Abstract
Eddy current separation (ECS) is a process used throughout the scrap recycling industry for sorting nonferrous metals from other nonmetallic fluff. The mechanism is based on the principle that a time-varying magnetic field will induce electrical current to flow throughout the body of a conductive particle. This current then reacts to the applied magnetic field by exhibiting a pronounced force of deflection, thereby separating the materials. The process is remarkably efficient and environmentally friendly, making it essential to the growth and sustainability of the metal recycling industry. In this review, we aim to summarize the available literature on eddy current separators for recovery of nonferrous metals. Although several different designs of eddy current separators have been examined through the years, the most common in use today remains the belt-driven rotary drum design. Limitations of the belt-driven rotary drum design are discussed as well as potential new designs and new applications for eddy current separators.
Introduction
Metals play an integral role in modern society. In 2017 alone, the apparent U.S. consumption of iron and steel reached 100 million metric tons (USGS, 2018). Aluminum consumption likewise reached almost six million metric tons, with copper following at 1.85 million metric tons (USGS, 2018). Unfortunately, the mining and processing of such metals can have large environmental impacts if not managed properly, such as destruction of native landscapes, acid mine drainage/wastewater treatment, and gaseous and particulate emissions. Increasing consumer demand also contributes heavily to increasing environmental impacts. Relative to production rates in the 1900s, six out of 11 of the most commonly recovered metals are now produced at rates of 10 or even 1000 times more (Johnson et al., 2007). To compound the problem even further, the ore grade of most operational mines has been steadily degrading over time. For example, the global average ore grade of copper has decreased from 1.1 wt% in the 1970s to about 0.8 wt% in 2009 (Crowson, 2012). Thus, to maintain consistent production levels, a larger amount of material must be mined and processed accordingly, which only results in further environmental impacts (Norgate and Haque, 2010, Norgate and Jahanshahi, 2010).
With the depletion of high-grade ores and an increasing global demand, we are faced with two possible solutions: (1) improve our current extraction methods of low-grade virgin ore deposits, or (2) develop and integrate new recycling technologies within the existing value chain. Due to the relative technological maturity of the mining industry, however, the first solution is likely to expect only incremental improvements in the foreseeable future. In contrast, recycling is a comparatively young industry with tremendous potential for growth and technological innovation. This is also evident from consistent economic growth that the recycling industry has demonstrated. For example, the U.S. metal scrap recycling industry has increased by 37% from $77 billion in 2010 to $106 billion in 2015 (ISRI, 2016). Another strong economic incentive for recycling is a study by Johnson et al. (2007) which found that most metals currently targeted for recycling have post-disassembly concentrations that are more enriched than minimum profitable ore grades. A recent study by Sverdrup et al. (2017) also estimates that scrap will become the main source for iron, aluminum, and copper within the next 30 years.
Of all the materials used in society, metals have the greatest recycling potential. Not only are metals relatively easy to recover from municipal waste, but their physical properties do not degrade over time like other materials (e.g., plastics and paper). Recycling also requires significantly less energy than mining, since materials generally need only to be melted rather than processed from raw mineral deposits. For many common commodity metals (i.e., Al, Cu, Fe and steel), these savings can typically reach as high as 70% or more (Cui and Forssberg, 2003). Due to the high demands of electrolytic reduction, aluminum recycling can even reach as high 95% energy savings when compared to mining.
Based on U.S. Geological Survey data from 2015, the United States recycled approximately 58.3 Mt of the metals (Table 1), which was equivalent to about 49% of the supply of those metals (USGS, 2017). By percentage, the top three recycled metals were lead, titanium, and magnesium. By mass, however, 90% of the recycled metal was iron and steel. The recycling rates from 2011 to 2015 for the metals in Table 1 are plotted in Fig. 1. Generally speaking, recycling rates in the U.S. have either remained relatively constant or decreased slightly from 2011 to 2015, with the exception of titanium. Only chromium and nickel experienced a noticeable increase in recycling rates from 2014 to 2015. Despite the numerous advantages to metal recycling, the supply of nonferrous metals in the United States tends to vary between 30 and 60% from recycled sources.
In order to effectively recycle scrap metal, it must first be separated from other materials (e.g., polymers/plastics, glass, fibrous materials, construction materials, etc.), as well as other dissimilar metals (e.g., ferrous from nonferrous). This process is arguably one of the most important cost barriers to the entire industry, as it depends highly on the availability of technology as well as the composition of a particular stream. Similar to mineral processing, the first step is usually some physical size reduction via chopping, shredding, crushing, etc. After size reduction, various physical separation methods can be used to separate the mixture based on properties of the material. This is accomplished by exploiting differences in physical properties of various materials such as density, magnetism, and electrical conductivity.
A metal shredder plant generally generates three fractions, commonly referred to as the Ferrous, Heavy Fraction (HF), and Light Fraction (LF). The Ferrous mainly consists of iron and steel products, and is separated by magnetic separators after shredding. The HF consists of mainly of nonferrous metals, alloys, and nonmetallic materials. The LF is mainly dirt/fluff with a small metal fraction (less than 5%) and is recovered by screening and air suction.
Automotive Shredder Residue (ASR) is a combination of HF and LF, and annually generates approximately five million tons worldwide, which is about 5% of the global industrial waste (Mallampati et al., 2018). Most of this residue ends up in landfills or is converted thermally (Cossu and Lai, 2015, Nicolli et al., 2012, Simic, 2013). Automotive Shredder Residue consists of a wide variety of materials, including precious metals, plastics, glass, rubber, wood, foam, tramp metal, wire, fibers, sand, and dirt (Cossu and Lai, 2015, Simic, 2013, Widmer et al., 2015). The compositions of these fractions are dynamic and highly dependent on the feed materials. Generally, the Ferrous fraction can be sold directly to steel producers without much further purification/treatment, whereas the HF requires further purification before it can be sent to metal producers. The LF is often landfilled and has been a topic of research to recover engineered polymers, energy, and metals.
Refining of the HF requires the further use of separation methods/technologies to acquire an enriched product. Sometimes to achieve a desired quality fraction, even further size reduction may be required. Although nonmetallic materials are generally still within the HF, these can be removed by density separation methods. For smaller particles (< 0.5 mm) in the LF, froth flotation can be utilized to further recover engineered polymers of value.
Ferrous scrap metal is that which contains iron, and is generally removed first is scrap sorting. Examples of some common ferrous metal scrap include alloy steel, carbon steel, cast and wrought iron. It is worth noting that a common misnomer is to associate ferrous with magnetic and vice versa. Ferromagnetism is a magnetic property, which describes how certain materials form permanent magnets or are attracted to magnets. Examples of ferromagnetic materials include iron, nickel, cobalt, some rare earth oxides, as well as some naturally occurring minerals, e.g., lodestones (magnetite is actually considered ferrimagnetic, which is similar to ferromagnetic). Other types of magnetism include paramagnetism, diamagnetism, and antiferromagnetism, but are generally much weaker effects.
Magnetic separation is a process that extracts ferromagnetic materials through the use of powerful, permanent magnets. The basic principle has been also used since the mid-1800s in mineral processing for removal or tramp iron or iron ore beneficiation. It is also used to remove strongly ferromagnetic impurities from non-metallic ore (e.g. quartz and feldspar). A review by Oberteuffer (1974) covers the of principles, devices, and applications of magnetic separation. Although magnetic separators utilize magnetic properties to separate materials, they do not work particularly well with weakly magnetic or non-ferromagnetic metals. For example, aluminum and titanium are nonferrous metals that are technically magnetic. However, they do not have strong innate magnetic moments, and thus a different separation mechanism is required.
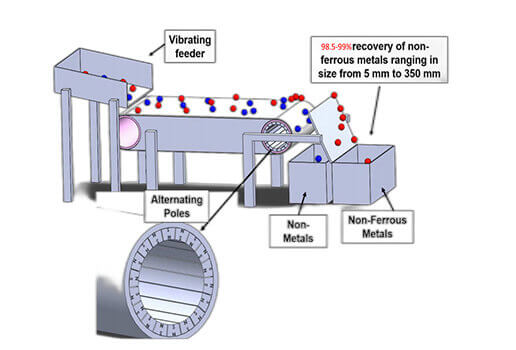
After magnetic separation has removed the bulk ferrous fraction from a scrap material stream, the most common processing step to occur next is eddy current separation (ECS). The mechanism works by exposing conductive, nonferrous particles to a time-varying magnetic field, which in turn gives rise to electrical currents throughout their volumes (Schloemann and Reiner, 1979). The relative motion between the current and the magnetic field gives rise to a force, called the Lorentz force, which subsequently deflects the nonferrous scrap particles away from the nonmetallic fluff. A Lorentz force is the combination of electric and magnetic force on a particle due to electromagnetic fields.
In most commercial designs, the time-varying magnetic field is achieved by rotating a cylindrical array of permanent magnets with alternating polarity (Fig. 2). If the magnetic array is both strong enough and fast enough, metallic particles will actually lift off the feed belt and hurl over the mechanical splitter nearby. The process tends to work well at extracting particles of aluminum, copper, brass, and zinc, and can even do so with a throughput of many tons per hour. In contrast, the attractive forces on ferrous materials are usually much stronger than the repulsive Lorentz forces, which is why magnetic separation is a common pre-processing step that always occurs before ECS.
The application of ECS technology offers a robust method to separate a nonferrous fraction with high recovery; however, existing ECS designs are limited by particle geometry. Although eddy current phenomenon was discovered well over a hundred years ago and is used in the design of electric motors, dynamos, and transformers, for example, there still exist knowledge gaps in the fundamental understanding of eddy currents. In regards to eddy-current separators, many developments and U.S. patents were granted in the 1960s. Further developments where contributed by Schloemann, Forssberg, Rem, and Zhang from the late 1970s to the 1990s. Eddy current separation has historically been overlooked as a means of separating nonferrous metals from a mixture of other materials. The designs of these separators have only made incremental advancements in metal fraction separations, while new designs of eddy current separators are stagnant. This is a result of a technical understanding of the separation mechanism. The available literature on the subject is still in development. Reviews on the technical applications of eddy current separators have been published by Schloemann, 1979, Schloemann, 1982) and more recently by Jujun et al., 2014, Wang et al., 2013. However, the number of extensive and critical reviews on this topic is limited to date.
Section snippets
Theory of eddy current separation
Although the basic principle of eddy current separation has been well known for decades, the underlying theory that governs it is notoriously complex. An excellent text to review the formulation of electromagnetic fields in materials is that by Jackson (1999). Broadly speaking, the process begins with a time-varying magnetic field intensity B(r,t), where r is an arbitrary position vector and t is time. According to Faraday’s law, the time-variation on B gives rise to an electric field E in
Current commercial offerings of eddy-current separators
Despite the variety of research and commercial ECSs, all of the current ECS companies offer minor variations of the conventional rotary-drum type of separator (i.e., mechanical movement of permanent magnets). This type of separator has established itself as the default in this industry. The design (Fig. 2) essentially consists of a belt conveyor with its drive at the feed end and a high-speed rotor with permanent magnets of alternating polarity at the discharge end. The magnetic rotor,
Historical survey of eddy current separation technology
The first record of observing eddy currents, which was called “rotatory” magnetism, and the fact that most nonferrous conductive bodies could be magnetized, was reported by François Arago in 1824 (Baily, 1879). The phenomenon, by which electrical currents appear in any conductor when exposed to a time varying magnetic field, or rather, electromagnetic induction, was later further explained by Faraday in 1831 (Anderson, 1993). In 1834, Heinrich Lenz pronounced his law that the induced current in
Future challenges and direction
Having reviewed the different types of eddy-current separators (ECS) described in the scientific and technical literature for the last 130 some years, in this section we aim to discuss future challenges and potential directions for development in ECS research. The design and performance of the rotating belted-drum type eddy current separators have been improved substantially in the last ten years, primarily due to advances in magnet materials and magnet configurations, as well as a better
Conclusion
In this review we have made an attempt to summarize the current available literature on eddy current separators for recovery of nonferrous metals from waste streams. Many technological breakthroughs have enabled the industrial scale use of such systems in metal scrap sorting and recycling scenarios. However, there still remains knowledge gaps in the fundamental understanding the of separation mechanism. Moreover, the ability to model such systems remains a technical challenge with substantial